
A unit plan on the particle nature of light and modern physics
- Subject:
- Physics
- Science
- Material Type:
- Unit of Study
- Provider:
- Fermi National Lab
- Author:
- Amanda Early
- Stacy Gates
- Date Added:
- 11/08/2024

A unit plan on the particle nature of light and modern physics

In this interactive activity from ChemThink, examine the basic properties of matter at an atomic level and consider how various atoms affect the way a substance behaves.

Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.

Explore the interactions between various combinations of two atoms. Observe the the total force acting on the atoms or the individual attractive and repulsive forces. Customize the attraction to see how changing the atomic diameter and interaction strength affects the interaction.
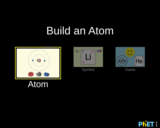
Build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. Then play a game to test your ideas!

Explore molecule shapes by building molecules in 3D. Find out how a molecule's shape changes as you add atoms to a molecule.

Students studying molecule shapes will better understand its concepts with this virtual experiment that tests how a molecule's shape changes due to repulsions between atoms. Learning is further enhanced by building molecules in 3D.

When do photons, electrons, and atoms behave like particles and when do they behave like waves? In this interactive, use quantum detectors to explore how measurements change the waves and the patterns they produce on the screen.

After watching this simulation, students will be able to describe characteristics of the three states of matter. They will watch as atoms and molecules change between solid, liquid and gas phases.

An interactive simulation that teaches about quantum mechanics, spin, and quantum measurement through a classic Stern-Gerlach Experiment. By observing the spin of atoms as an intrinsic angular momentum, students learn to measure to reach a value of spin up or spin down. This simulation can either be downloaded or played online and includes handouts, lesson plans, and additional materials.

Describe the difference between the subatomic particles, including their masses, locations, and charges. This lesson is 5 of 7 in the series titled "Subatomic Particles."

Electron orbitals. Diagonal rule. Dot diagrams. The periodic table. There's so much to know about the tiniest building blocks of life- atoms. [4:41]

George Zaidan and Charles Morton shape our image of molecules. [3:47]

Dr. Chris Muhlstein explains the challenge of studying materials that are too small to see with the naked eye. The technique some scientists use to observe individual atoms is similar to the technique of using touch to find out the size, shape, and location of objects in a dark room. By using a very small, sharp sensor, scientists can create an image of atoms. [1:17]

A discussion of how Lucretius's view of matter as composed of individual atoms preceeded modern thought by almost 2000 years.

Looks at the history of atomic theory and ideas about atomic models that have evolved over time. Covers models put forward by John Dalton, J.J. Thomson, Earnest Rutherford, Niels Bohr, and the work of Schrodinger and Heisenberg. Also discusses photons and the photoelectric effect. [16:12]
This lesson is introductory for electrical students. I used a worksheet and I altered a power point presentation from this Open Space lesson plan. I added my own written worksheet. So, it could be said that the lesson plan was remixed.

This video is for teachers to explore how to help students apply ideas about matter consistently across many scientific disciplines. [8:37]