
In this video segment, the ZOOM cast demonstrates how to use cabbage juice to find out if a solution is an acid or a base.
- Subject:
- Science
- Material Type:
- Audio/Video
- Lesson
- Provider:
- PBS LearningMedia
- Date Added:
- 11/06/2023

In this video segment, the ZOOM cast demonstrates how to use cabbage juice to find out if a solution is an acid or a base.

What happens when you mix baking soda and lemon juice? Watch the ZOOM cast launch a rocket using kitchen chemistry. [3:26]
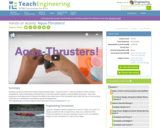
In this activity, students construct their own rocket-powered boat called an "aqua-thruster." These aqua-thrusters will be made from a film canister and will use carbon dioxide gas produced from a chemical reaction between an antacid tablet and water to propel it. Students observe the effect that surface area of this simulated solid rocket fuel has on thrust.

Have you ever enjoyed watching something lift off into the air, like fireworks at a show or a spacecraft launching? It can be an amazing experience. It is thrilling to see something lift off against Earth's gravity. To launch a spacecraft, its rockets give it a strong push that is due to a chemical reaction. This means that every time you see a spacecraft launch, you are watching chemistry at work. In this activity you will get to blast an object into the air using two simple ingredients -- baking soad and vinegar. Investigate how to mix these ingredients to get the best lift off, and then you could give your friends and family a homemade, gravity-defying show!

Biology 2e is designed to cover the scope and sequence requirements of a typical two-semester biology course for science majors. The text provides comprehensive coverage of foundational research and core biology concepts through an evolutionary lens. Biology includes rich features that engage students in scientific inquiry, highlight careers in the biological sciences, and offer everyday applications. The book also includes various types of practice and homework questions that help students understand—and apply—key concepts. The 2nd edition has been revised to incorporate clearer, more current, and more dynamic explanations, while maintaining the same organization as the first edition. Art and illustrations have been substantially improved, and the textbook features additional assessments and related resources.


By the end of this section, you will be able to do the following:
Define matter and elements
Describe the interrelationship between protons, neutrons, and electrons
Compare the ways in which electrons can be donated or shared between atoms
Explain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms

Rockets need a lot of thrust to get into space. In this lesson, students learn how rocket thrust is generated with propellant. The two types of propellants are discussed and relation to their use on rockets is investigated. Students learn why engineers need to know the different properties of propellants.

Paul Andersen explains how atoms are conserved in a chemical reaction. This can be seen in a chemical equation where the subscripts represent the atoms in the molecule and the coefficients represent the molecules. The mass of an anylate can be determined through both gravimetric analysis and a titration. [12:18]

In this video Paul Andersen explains that elementary reactions are steps within a larger reaction mechanism. Colliding molecules require sufficient energy and proper orientation to break bonds and form new bonds. A unimolecular reaction mechanism requires one type of reactant and is a first-order reaction. Enhance learning with the additional resources. [6:13]

Paul Andersen explains how an overall chemical reaction is made up of several elementary steps. The stoichiometry of this equation can be predicted but the rate law must be measured. If the elementary steps of the reaction are determined the rate law of this individual step may be predicted. Don't miss the concept map and slideshow. [4:03]

Use this narrated tutorial to help understand how to balance a chemical equation using coefficients and leaving formula subscripts unchanged. [9:35]

Watch this screencast introducing several common types of chemical reactions. [1:36]

A video lesson which illustrates how to identify and complete a synthesis chemical reaction. [8:08]

This lesson summarizes how chemical reactions occur, and that not all reactions go to completion. [1:41]
Students explore the science of microbial fuel cells (MFCs) by using a molecular modeling set to model the processes of photosynthesis and cellular respiration—building on the concept of MFCs that they learned in the associated lesson, “Photosynthesis and Cellular Respiration at the Atomic Level.” Students demonstrate the law of conservation of matter by counting atoms in the molecular modeling set. They also re-engineer a new molecular model from which to further gain an understanding of these concepts.

Students create silver nanoparticles using a chemical process; however, since these particles are not observable to the naked eye, they use empirical evidence and reasoning to discover them. Students first look for evidence of a chemical reaction by mixing various solutions and observing any reactions that may occur. Students discover that copper and tannic acids from tea reduce silver nitrate, which in turn form silver. They complete the reaction, allow the water to evaporate, and observe the silver nanoparticles they created in plastic dishes using a stereo microscope. Students iterate on their initial process and test to see if they can improve the manufacturing process of silver nanoparticles.
Students acquire a basic understanding of the science and engineering of space travel as well as a brief history of space exploration. They learn about the scientists and engineers who made space travel possible and briefly examine some famous space missions. Finally, they learn the basics of rocket science (Newton's third law of motion), the main components of rockets and the U.S. space shuttle, and how engineers are involved in creating and launching spacecraft.

Create a giant foaming reaction and wow your friends with the classic science demonstration! With just a few simple ingredients, you can make something that looks like toothpaste being squeezed from a tube -- but so big, it must be for elephants!