
Watch this video lesson introducing the atom, and learn about its unique structure. [3:58]
- Subject:
- Science
- Material Type:
- Audio/Video
- Provider:
- Sophia Learning
- Date Added:
- 12/01/2023

Watch this video lesson introducing the atom, and learn about its unique structure. [3:58]

This video segment adapted from A Science Odyssey takes a look at the scale of the atom and the tremendous amount of space between the electrons and the nucleus. If all this empty space exists in matter, how can any substance be solid?

This video segment adapted from A Science Odyssey takes a look at the scale of the atom and the tremendous amount of space between the electrons and the nucleus. If all this empty space exists in matter, how can any substance be solid? [1:15]

Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a fine glossary. Shows how scientists? views of the fundamentals have changed over time. Wow!

Biology 2e is designed to cover the scope and sequence requirements of a typical two-semester biology course for science majors. The text provides comprehensive coverage of foundational research and core biology concepts through an evolutionary lens. Biology includes rich features that engage students in scientific inquiry, highlight careers in the biological sciences, and offer everyday applications. The book also includes various types of practice and homework questions that help students understand—and apply—key concepts. The 2nd edition has been revised to incorporate clearer, more current, and more dynamic explanations, while maintaining the same organization as the first edition. Art and illustrations have been substantially improved, and the textbook features additional assessments and related resources.


By the end of this section, you will be able to do the following:
Define matter and elements
Describe the interrelationship between protons, neutrons, and electrons
Compare the ways in which electrons can be donated or shared between atoms
Explain the ways in which naturally occurring elements combine to create molecules, cells, tissues, organ systems, and organisms
This lesson discusses the result of a charge being subject to both electric and magnetic fields at the same time. It covers the Hall effect, velocity selector, and the charge to mass ratio. Given several sample problems, students learn to calculate the Hall Voltage dependent upon the width of the plate, the drift velocity, and the strength of the magnetic field. Then students learn to calculate the velocity selector, represented by the ratio of the magnitude of the fields assuming the strength of each field is known. Finally, students proceed through a series of calculations to arrive at the charge to mass ratio. A homework set is included as an evaluation of student progress.

Paul Andersen explains how electric charge is quantized and how the smallest unit of charge is 1.6x10^-19 C, or the elementary charge. [4:51]

In the following video the wave model of the electron can be used to explain the Bohr model. Electrons are found in certain orbits because they interfere with themselves and create standing waves. When the wavelengths don't match up with a whole integer they will create destructive interference. [4:02]
Students learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.
Working in teams of three, students perform quantitative observational experiments on the motion of LEGO MINDSTORMS(TM) NXT robotic vehicles powered by the stored potential energy of rubber bands. They experiment with different vehicle modifications (such as wheel type, payload, rubber band type and lubrication) and monitor the effects on vehicle performance. The main point of the activity, however, is for students to understand that through the manipulation of mechanics, a rubber band can be used in a rather non-traditional configuration to power a vehicle. In addition, this activity reinforces the idea that elastic energy can be stored as potential energy.

Acting as part of an overview on quantum theory, this section of the site deals with questions related to electrons, such as "If the electron cannot be localized, can it be moving?" and "Why does the electron not fall into the nucleus?"

Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site seeks to answer the question, "Why doesn't the electron fall into the nucleus?"

Here you can find some great information about the 9th element in the periodic table, "fluorine." Content focuses on fluorine's electrons, where you can find fluorine in nature and in the home, and how fluorine combines with other elements.
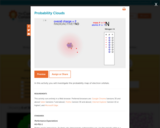
In this interactive activity, learners build computer models of atoms by adding or removing electrons, protons, and neutrons. It presents the orbital model of an atom: a nucleus consisting of protons and neutrons with electrons surrounding it in regions of high probability called orbitals. Guided tasks are provided, such as constructing a lithium atom and a carbon-12 atom in the fewest possible steps. The activity concludes with a model for building a charged hydrogen atom (an ion). Within each task, students take snapshots of their work product and answer probative questions. This item is part of the Concord Consortium, a nonprofit research and development organization dedicated to transforming education through technology.
This lesson introduces students to the fundamental concepts of electricity. This is accomplished by addressing questions such as "How is electricity generated," and "How is it used in every-day life?" The lesson also includes illustrative examples of circuit diagrams to help explain how electricity flows.

An excellent overview of electron spin, what it means in non-mathematical terms and quantum mechanical terms.
This StudyCards stack enables students to review the vocabulary used in studying electron behavior.