
This video and slideshow provides an overview and demonstration of the balances and glassware commonly used to prepare solutions in a laboratory. [24.08]
- Subject:
- Science
- Material Type:
- Audio/Video
- Provider:
- BioEd Online
- Date Added:
- 07/01/2022

This video and slideshow provides an overview and demonstration of the balances and glassware commonly used to prepare solutions in a laboratory. [24.08]

Biology 2e is designed to cover the scope and sequence requirements of a typical two-semester biology course for science majors. The text provides comprehensive coverage of foundational research and core biology concepts through an evolutionary lens. Biology includes rich features that engage students in scientific inquiry, highlight careers in the biological sciences, and offer everyday applications. The book also includes various types of practice and homework questions that help students understand—and apply—key concepts. The 2nd edition has been revised to incorporate clearer, more current, and more dynamic explanations, while maintaining the same organization as the first edition. Art and illustrations have been substantially improved, and the textbook features additional assessments and related resources.


By the end of this section, you will be able to do the following:
Explain why and how passive transport occurs
Understand the osmosis and diffusion processes
Define tonicity and its relevance to passive transport


By the end of this section, you will be able to do the following:
Describe the properties of water that are critical to maintaining life
Explain why water is an excellent solvent
Provide examples of water’s cohesive and adhesive properties
Discuss the role of acids, bases, and buffers in homeostasis
Students learn about various crystals, such as kidney stones, within the human body. They also learn about how crystals grow and ways to inhibit their growth. They also learn how researchers such as chemical engineers design drugs with the intent to inhibit crystal growth for medical treatment purposes and the factors they face when attempting to implement their designs. A day before presenting this lesson to students, conduct the associated activity, Rock Candy Your Body.
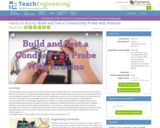
Student groups construct simple conductivity probes and then integrate them into two different circuits to test the probe behavior in solutions of varying conductivity (salt water, sugar water, distilled water, tap water). The activity culminates with student-designed experiments that utilize the constructed probes. The focus is to introduce students to the fabrication of the probe and expose them to two different ways to integrate the probe to obtain qualitative and quantitative measurements, while considering the application and utility of a conductivity probe within an engineering context. A provided handout guides teams through the process: background reading and questions; probe fabrication including soldering; probe testing and data gathering (including circuit creation on breadboard); probe connection to Arduino (including circuit creation and code entry) and a second round of testing and data gathering; design and conduct their own lab experiments that use the probes; online electrolyte/nonelectrolyte reading, short video, comprehension check and analysis questions.
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use alcohol and chromatography paper to separate the components of black and colored marker ink. Students witness first-hand how components of a solution can be separated, even when those individual components are not visible in solution.
Students investigate the property dependence between concentrations and boiling point. In section 1, students first investigate the boiling point of various liquid solutions. In section 2, they analyze data collected by the entire class to generate two boiling point curves, one for salt solutions and one for sugar solutions. Finally, in section 3, students use the data they have analyzed to determine how to create a solution that has a particular boiling point and is a cost-effective design.

Students experiment with various ways to naturally dye materials using sources found in nature—roots, leaves, seeds, spices, etc.—as well as the method of extracting dyes. Then they analyze various materials using statistical methods and tackle an engineering design challenge—to find dyes that best suit the needs of a startup sustainable clothing company.
Students gain an understanding of the difference between electrical conductors and insulators, and experience recognizing a conductor by its material properties. In a hands-on activity, students build a conductivity tester to determine whether different objects are conductors or insulators. In another activity, students use their understanding of electrical properties to choose appropriate materials to design and build their own basic circuit switch.

Students learn how crystallization and inhibition occur by examining calcium oxalate crystals with and without inhibitors that are capable of altering crystallization. Kidney stones are composed of calcium oxalate crystals, and engineers and doctors experiment with these crystals to determine how growth is affected when a potential drug is introduced. Students play the role of engineers by trying to determine which inhibitor would be the best for blocking crystallization.
This lesson plan introduces the properties of mixtures and solutions. A class demonstration gives the students the opportunity to compare and contrast the physical characteristics of a few simple mixtures and solutions. Students discuss the separation of mixtures and solutions back into their original components as well as different engineering applications of mixtures and solutions.

This lesson will provide an understanding of the chemical and physical nature of water. It is 1 of 4 in the series titled "The Properties of Water."
Students see and learn how crystallization and inhibition occur by making sugar crystals with and without additives in a supersaturation solution, testing to see how the additives may alter crystallization, such as by improving crystal growth by more or larger crystals. After three days, students analyze the differences between the control crystals and those grown with additives, researching and attempting to deduce why certain additives blocked crystallization, showed no change or improved growth. Students relate what they learn from the rock candy experimentation to engineering drug researchers who design medicines for targeted purposes in the human body. Conduct the first half of this activity one day before presenting the associated lesson, Body Full of Crystals. Then conduct the second half of the activity.

Water is essential for life as we know it, due in part to its ability to dissolve a wide range of substances. In fact, most chemical reactions occur in a dissolved state. This pathway provides resources regarding how water is able to act as a solvent.

Build your own lightsaber